
HYDERABAD, MARCH 21, 2024: Officials of Telangana Drugs Control Administration have seized tablets for misleading advertisement, overpriced steroid injections and other tablets falsely manufactured and sold as food products in various parts of the state on Wednesday and Thursday.
DCA Director-General VB Kamalasan Reddy said that detected a medicine ‘‘Colinol-SPAS Tablets,’ circulating in the market with misleading claim on its label that it treats ‘disorders of menstrual flow’, which is a contravention of the Drugs and Magic Remedies (Objectionable Advertisements) Act, 1954. The said medicine was detected by DCA officials at Konark Pharma Distributors, Kamareddy. Stocks were seized during the raid.
The advertisement of a medicine for the treatment of ‘disorders of menstrual flow’ in general is prohibited under the Drugs and Magic Remedies (Objectionable Advertisements) Act, 1954. Raja Reddy, Drugs Inspector, Kamareddy carried out the raid.


In Badradri Kothagudem, the officials seized ‘Dexalab’ (Dexamethasone Injection I.P.) which is under price control as per Drugs (Prices Control) Order, 2013 and the price of product shall be in accordance with the ‘Ceiling Price’ fixed by National Pharmaceutical Pricing Authority (NPPA), Government of India. The product seized ‘Dexalab’ (Dexamethasone Injection I.P.) bears MRP as Rs. 39.90 per 30 ml on the label of the product which is a violation of Drugs (Prices Control) Order, 2013. The Ceiling price fixed by the Central Government including Wholesale Price Index (WPI) for the product is Rs. 0.89 per 1 ml i.e. Rs. 26.7 per 30 ml (Ceiling Price).
Hence Maximum Retail Price (MRP) i.e. including GST 12 % should not be more than Rs. 29.904 per 30 ml (MRP=Ceiling Price+GST). The firm overpriced the product. Firm charged excess of Rs. 9.996 per 30 ml which is a violation of Drugs (Prices Control) Order, 2013. G. Prasad, Assistant Director, Khammam, Ch. Sampath Kumar, Drugs Inspector, Bhadradri-Kothagudem are among the officers who carried out the raid.
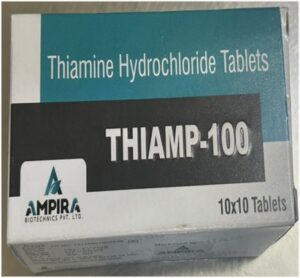
In another incident in Malkajgiri, the officials acting on gathered intelligence, have detected the product ‘THIAMP-100 tablets’ (Thiamine Hydrochloride Tablets 100 mg) circulating in the market. These tablets were falsely manufactured under a ‘food license (FSSAI license)’ and falsely claimed to be a food product/nutraceutical. The tablets were found to be manufactured at Vatave Healthcare, SAS Nagar, Pabhat-140603, Punjab and were being illegally marketed by Ampira Biotechnics Pvt. Ltd., Chandigarh-160101, as a food product/nutraceutical.
The label of the product ‘THIAMP-100 tablets’ claims to contain Thiamine Hydrochloride 100 mg, which is classified as a ‘drug’ used to treat ‘Vitamin B1’ deficiency according to the Drugs and Cosmetics Act. The product ‘THIAMP-100 tablets’ (Thiamine Hydrochloride Tablets 100 mg) must be manufactured only under a ‘drug license’ issued under the Drugs and Cosmetics Act, adhering strictly to the ‘Good Manufacturing Practices’ (GMP) outlined in Schedule-M of the Drugs Rules. Additionally, it must meet the quality standards prescribed in the ‘Indian Pharmacopoeia’ (IP) as mandated. K. Murali Krishna, Drugs Inspector, Malkajgiri and T. Shiva Teja, Drugs Inspector, Kapra are among the officers who carried out the raid.















